Dr Sasha Howard, MBBS, MSc, MRCPCH, PhD

Wellcome Trust Clinical Research Career Development Fellow, Clinical Reader and Honorary Consultant in Paediatric Endocrinology
Centre: Endocrinology
Email: s.howard@qmul.ac.ukTelephone: +44(0) 20 7882 6235Twitter: @drsashahoward
Profile
ORCID ID: 0000-0002-6698-903X
Dr Sasha Howard graduated in Medicine from the University of Cambridge (preclinical medicine, 1st class honors) and University College London (MBBS, distinction) in 2004. She trained in Paediatrics before sub-specialising in Paediatric Endocrinology at Barts Health, UCLH and Great Ormond Street Hospitals. She completed a PhD in Molecular Endocrinology at the William Harvey Research Institute, Barts and the London School of Medicine funded by a Wellcome Trust Research Training Fellowship and a Barts Charity Clinical Training Fellowship. She was awarded the 2015 Henning Andersen prize for Paediatric Endocrinology (ESPE) and 2016 1st Oral Plenary Prize at the Academy of Medical Sciences Spring Meeting for Clinician Scientists. In 2017 she won a 4-year NIHR clinical lectureship alongside an Academy of Medical Sciences starter grant to continue her research into disorders of human pubertal timing. She was awarded a Wellcome Trust Clinical Research Career Development Fellowship, stage 1 in 2021 and appointed Senior Lecturer at Queen Mary University of London and Honorary Consultant in Paediatric Endocrinology at Barts Health NHS Trust. In August 2024, she was promoted to Clinical Reader (Associate Professor).
Dr Howard is Integrated Academic Training lead for the Specialised Foundation Programme, QMUL. Dr Howard is senior lead for the pan-London paediatric research network REACH. She is a member of the European Society for Paediatric Endocrinology (ESPE) programme organising committee and Paediatric and Adolescent Gynaecology Working Group, and of the NIHR / BSPED Clinical Studies Group (CSG) and Co-PI of the Barts Pituitary Centre.
Memberships
- The Royal College of Paediatrics and Child Health (RCPCH)
- British Society of Paediatric Endocrinology and Diabetes (BSPED)
- British Society of Paediatric Endocrinology and Diabetes (BSPED) Clinical Studies Group (CSG)
- European Society of Paediatric Endocrinology (ESPE)
- The Society for Endocrinology (SfE)
- American Endocrine Society (ENDO)
- British Medical Association (BMA)
Awards
- 2024 NIHR Efficacy and Mechanism Evaluation grant
- 2024 HARP Wellcome DTP Fellowship to Dr R Varughese
- 2023 Rosetrees Trust Major Project grant
- 2023 ESPE Research Unit
- 2023 ESPE Early Career Scientific Development Grant Dr F D’Aniello
- 2023 Society for Endocrinology, Early Career Grant to Dr J Read
- 2023 British Society of Neuroendocrinology, Project Grant to Dr J Read
- 2023 Isaac Shapera Medical Trust, IAT award for SFP to Dr Y Aung
- 2023 NIHR IAT award for ACF to Dr E Alexander
- 2023 FinRett Project Grant
- 2022 British Society of Neuroendocrinology, Student Project Grant
- 2021 British Society of Paediatric Endocrinology and Diabetes Research and Development Award
- 2021 British Society of Neuroendocrinology, Project Grant
- 2021 Wellcome Trust Clinical Research Career Development Fellowship Stage 1
- 2021 Barts Charity Large Programme Grant
- 2018 Academy of Medical Sciences CL Starter Grant
- 2018 Rosetrees Trust Award
- 2017 NIHR Academic Clinical Lectureship
- 2014 Wellcome Trust Pre-Doctoral Research Training Fellowship
- 2012 Rosetrees Trust Award
- 2012 Barts and the London Charity Clinical Training Fellowship
Find out more about Endocrinology at the William Harvey Research Institute.
Research
Group members
- Dr Jordan Read (PDRA)
- Dr Yasmin Al-Sayed (PDRA)
- Dr Charlotte Hall (PDRA Bioinformatician
- Mr Saleh Momeni (PhD student)
- Dr Rachel Varughese (Clinical Research Fellow)
Close collaborators
- Dr Leonardo Guasti (Reader in Endocrinology)
- Professor Leo Dunkel (Professor of Paediatric Endocrinology)
- Dr Alessia David (Imperial College London)
- Dr Ali Abbara (Imperial College London)
- Professor Mehul Dattani (Great Ormond Street Hospital for Children and ICH-UCL)
Alumni
- Dr Tansit Saengkaew, (PhD Student, now Assistant Professor of Paediatric Endocrinology and Medical instructor, Prince of Songkla University, Thailand)
- Dr Alessandra Mancini (PhD student, now postdoc at Harvard University)
- Ms Matilde Ciaroni (Erasmus student, now in Industry)
- Dr Yuri Aung (SFP)
- Dr Kyla Ng Yin (SFP)
- Dr Emma Alexander (Academic Clinical Fellow)
- Dr Francesco D'Aniello (ESPE Early Scientific Career Fellow)
- Dr Sophie Rhys-Evans (Specialist Foundation Programme Fellow)
- Dr Sebastian Castro (ESPE Early Scientific Career Fellow)
Summary
Dr Howard’s research is focused on optimising the diagnosis and management of patients with pubertal disorders. She is clinically active as an Honorary Consultant Paediatric Endocrinologist at Barts Health, running specialist puberty services and have developed national guidelines for the management of pubertal disorders.
Puberty is the period of physical and psychological change from a child to an adult. Disorders of pubertal timing affect 4% of children, comprising significantly precocious (before 8yrs of age [>2 standard deviations below the mean population age]) or delayed (after 14yrs of age [>2 standard deviations above the mean age]) pubertal onset.
These common disorders are associated with adverse long-term health outcomes. Precocious puberty is associated with an increased risk of obesity, type 2 diabetes, and breast cancer, and delayed puberty is associated with psychosocial comorbidities and reduced bone density. Both are associated with early menopause or andropause.
While we know that these conditions are often inherited in families, we still understand very little about how they occur, and which gene changes are responsible. Currently, it can be difficult to distinguish which patients with early or late puberty need intensive treatment and follow up.
Genetics of Disordered Puberty Project (IRAS 95781; CPMS ID 30730, CI Dr Sasha Howard): our aim is to identify genetic mutations that cause Central Precocious Puberty (CPP) and Delayed Puberty (DP). To achieve this, we utilise various genetic testing methods to diagnose patients that have been referred to us. So far, our cohort of referrals stands at >130 patients in whom we have found a genetic diagnosis in 28%.
If you have a patient with suspected CPP or DP please get in contact and we will endeavour to provide you with a genetic diagnosis completely free of charge.
Important: We are a purely research-based group and therefore all genetic diagnoses may have to be validated by a NHS or nationally accredited facility.
Genetics of the timing of puberty
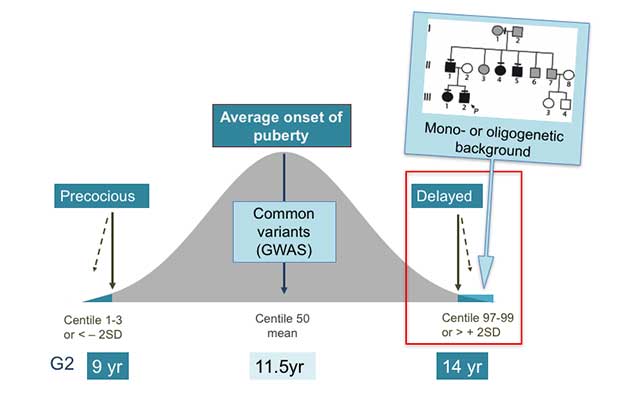
Figure 1. Genetics of the timing of puberty
The precise genetic causes of the extreme tails of normal puberty are unclear, as is the basis of association of specific gene variants with pubertal timing in the general population (outside of families with GnRH deficiency or self-limited delayed puberty). Understanding the role(s) of gene variants influencing the timing of puberty, both precocious and delayed, is expected to contribute to the understanding of the biological control of human pubertal timing both in disease and in the general population. This knowledge could directly help patients through improved diagnostic ease and facilitate identification of gene-environmental interactions. Based on the observed inheritance pattern, we hypothesize that families with inherited self-limited delay in growth and puberty are enriched for genetic variants that have high-impact on pubertal timing and, that these variants are amenable to discovery using modern molecular genetic tools. Recently, we have discovered evidence that such high impact variants may influence the timing of puberty, significantly delaying the onset of puberty in a subset of families.
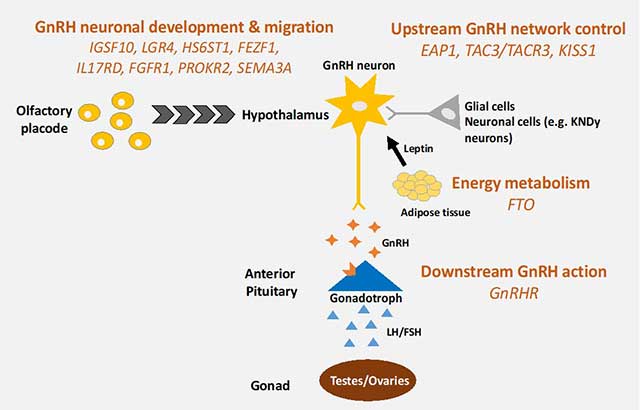
Figure 2. The genetic basis of delayed puberty
Previously identified genes implicated in the pathogenesis of self-limited delayed puberty are related to GnRH neuron development, up- and downstream GnRH function, and energy metabolism; KNDy - kisspeptin-neurokinin-dynorphin.
Reduced IGSF10 expression during embryogenesis in the corridor of nasal mesenchyme cells of the vomeronasal organ (VNO) leading to the olfactory bulbs delays the migration of gonadotropin-releasing hormone (GnRH) neurons to the hypothalamus. This reduction manifests in adolescence as a phenotype of delayed puberty due to abnormalities of GnRH neuronal network function.
Management of Central Hypogonadism in Children
The management of male infertility is very challenging, particularly in severe forms of hormone deficiency. These hormones are specific signals produced by the brain, which direct development of the testicles or ovaries. Patients who are unable to produce these signals (named ‘gonadotropin hormones’) have a condition called gonadotropin deficiency (GD).
For males with GD medical therapy for fertility is often unsuccessful. This is because hormones drive the development of the testicles in infancy and at puberty, a process vital to enable later sperm production. Despite adult hormone replacement, individuals may suffer infertility, in addition to chronic health and psychological issues.
Standard treatment for lack of puberty in adolescent males with GD is with testosterone. This helps with physical development, but not with testicular development or sperm production. Views of patient and their carers highlights the importance for young men with GD of adequate pubertal development, testicular maturity and the ability to make sperm.
In contrast, replacement of gonadotropin hormones enables physical maturity and the growth spurt, as well as specific testicular growth, to occur during puberty. These medications are currently used in adult patients with GD within fertility services, but can be used in puberty to allow reproductive development at the appropriate age.
PinG Study - Pubertal Induction with Gonadotropins in Adolescent and Young Adult Males with Gonadotropin Deficiency (IRAS 1009018; CPMS ID 58941, CI Dr Sasha Howard): PinG study, funded by an NIHR EME programme grant.
Currently, most clinicians will treat delayed or absent puberty in male adolescent patients with Gonadotropin Deficiency (GD) with a course of intramuscular testosterone. Whilst this therapy will promote physical growth, development of secondary sexual characteristics, increase penile size and lead to further development of the scrotum, it cannot facilitate development of the testes to allow spermatogenesis.
In contrast, therapy with gonadotropins is used to induce fertility in adult males with hypogonadotropic hypogonadism.
Therefore, there has been increasing interest in the use of gonadotropins in boys and young men with GD to aid testicular development and improve long-term fertility outcomes. This is supported by data in adult patients, but protocols and doses vary widely, with some centres using single gonadotropin regimes (mostly commonly human chorionic gonadotropin, hCG) and others recombinant (r) FSH + hCG, with little standardisation.
High-quality data from adolescents and young adults is lacking, particularly relating to which combination of gonadotropins are most appropriate for pubertal induction.
We hypothesise that combined gonadotropin treatment (with hCG and rFSH), as compared to single therapy (hCG alone), to induce puberty in males with partial GD will lead to improved testicular maturation, thus facilitating an increased capacity for spermatogenesis in adult life for these patients.
Additionally, in pre-pubertal males with severe GD, pre-treatment with rFSH during induction of puberty is hypothesised to lead to an increase in the Sertoli cell population, measurable as an increase in testicular volume and inhibin B, thus leading to an increased capacity for spermatogenesis in adult life for these more severely affected patients.
The PinG study is an open-label randomised controlled study, comparing different regimens of gonadotropin treatment to induce male puberty. The protocol is stratified according to disease severity, into those patients with partial CHH aged 12-35 years as described according to the inclusion criteria listed, and maximal testes volume ≥4mls, and patients with severe CHH aged 12-35 years as described according to the inclusion criteria and maximal testes volume <4mls.
The study has been designed to closely mirror standard hospital care for induction of male puberty with testosterone, and so participants will be expected to make a similar number of hospital visits each 4-6 months as in standard care. Participants will be recruited to the study from their hospital care and return to standard hospital care after 18-24 months of study participation.
The study will be open-label with the treatment arms require differing regimes and frequencies of administration (rFSH is 3 x per week, hCG is once or twice per week). As a result, the participants and the clinicians co-ordinating their care and follow-up visits will be aware of treatment allocation.
Recruitment is across 16 sites in England and Scotland and is due to start in September 2025.
Sponsors
Collaborators
Internal
- Dr Carles Gaston-Massuet (Endocrinology)
- Dr Helen Storr (Endocrinology)
- Prof Márta Korbonits (Endocrinology)
- Dr Evelien Gevers (Endocrinology)
- Prof Michael Barnes (Bioinformatics)
- Dr Claudia Cabrera (Bioinformatics)
External
- Anna Cariboni (Milan, Italy)
- Yee-Ming Chan (Boston, USA)
- Raja Brauner (Paris, France)
- Romina Grinspon and Rodolfo Rey (Buenos Aires, Argentina)
- Alexander Jorge and Ana Claudia Latronico (São Paolo, Brazil)
- Verónica Mericq and Paulina Merino (Santiago, Chile)
- Vincent Prevot (Lille France)
- Marco Bonomi (Milan, Italy)
- Alessia David (Imperial, London, UK)
News
-
Huffington Post - adrenarche explained https://www.huffingtonpost.co.uk/entry/adrenarche-explained-parent-advice_uk_68370c6ee4b09d67a28ce742
- Daily Mail - Kallmann Syndrome https://www.dailymail.co.uk/health/article-14706287/I-small-genitals-no-sex-drive-smell-meet-men-didnt-puberty-little-known-syndrome-affects-thousands.html
- Paediatric Endocrinology - Expert Reviews (Medwire Newsfocus)
- Faulty 'delayed puberty' gene found (BBC News Online)
- Genetic causes of very late puberty (The Academy of Medical sciences)
- When breast is not best (Charlotte Philby)
Teaching
QMUL
- Primary Supervisor for PhD students (2024-2028)
- Primary Supervisor for ACF and SFP doctors (2021-2025)
- Primary Supervisor for SSC4/ MSc students (2021-2025)
- Academic Advisor MBBS (2023-2025)
- PGDip/MSc Endocrinology, Lecturer (2016-2025)
- OSCE examiner/ MBBS & GEP interviewer (2016-2025)
- Undergraduate Endocrine physiology and biochemistry, Lecturer (2017-2025)
Ex-QMUL
- ESPE Connect Webinar Dec (2023)
- MedEA Webinar series Oct (2023)
- Anne Klibanski Visiting Lecture Series, Massachusetts General Hospital, USA (May 2023)
- Invited symposium speaker, ESPE Rome Italy (2022)
- Invited symposium speaker, BSPED, (November 2021)
